Researchers at a Massachusetts biotechnology company have been working for years to develop an injectable foam that can control severe internal bleeding. A recent translational research study has demonstrated the optimal human dose of the foam, according to a press release from Arsenal Medical, developer of the technology. The company expects to seek regulatory approval for the trauma foam system this year.
Temporary implant
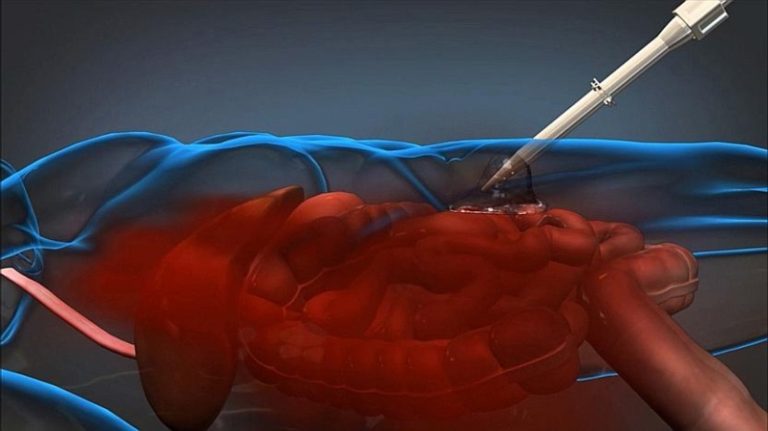
The trauma foam system is designed to treat non-compressible abdominal hemorrhage. The foaming agent is delivered percutaneously using a laparoscopic device. The goal is to give battlefield and prehospital teams enough time to transport patients with severe internal bleeding to a surgical facility.
According to the company, the trauma foam:
- Achieves conformal contact with injured tissues in complex anatomies, even in the presence of severe bleeding
- Has the ability to tamponade bleeding tissue in intricate spaces without direct visualization
- Uses biocompatible, well characterized materials
“The foam is actually a polyurethane polymer that forms inside a patient’s body upon injection of two liquid phases, a polyol phase and an isocyanate phase, into the abdominal cavity,” according to a press release from the Defense Advanced Research Projects Agency (DARPA). The mix expands to approximately 30 times its original volume, then transforms into solid foam. “The foam can expand through pooled and clotted blood and despite the significant hydrostatic force of an active hemorrhage.”
DARPA is an agency of the U.S. Department of Defense. It provided partial funding for development of the foam technology.
“In tests, removal of the foam took less than one minute following incision by a surgeon,” according to DARPA. “The foam was removed by hand in a single block, with only minimal amounts remaining in the abdominal cavity, and with no significant adherence of tissue to the foam.”
Human dose
The translational research study was conducted in 18 recently deceased humans, according to Arsenal Medical. The investigation took place at Massachusetts General Hospital, Oregon Health and Sciences University, and the University of Texas Health Science Center at Houston.
“Research teams injected the foam into the abdomen within three hours after the patients had passed away,” according to the company press release. “The study assessed the foam’s performance by monitoring the pressure it generated and how it contacted the organs.” The results of the study were reported in December at the Annual Scientific Assembly of the Eastern Association for the Surgery of Trauma (EAST).
“This investigation has allowed translation of a huge body of preclinical animal research, resulting in a dose identified as appropriate for humans,” said David R. King, MD, FACS, principal investigator. Dr. King is an attending trauma surgeon at Massachusetts General Hospital, an assistant professor of surgery at Harvard Medical School and a lieutenant colonel in the U.S. Army.
Multiple care scenarios
Arsenal Medical expects to file a regulatory submission for the trauma foam technology with the U.S. Food and Drug Administration (FDA) by mid-2015.
If approved for human use, the trauma foam could be used by civilian paramedics to treat patients in the field. In an earlier paper, Dr. King also raised the possibility of in-hospital use within a regional trauma system.
“One can also imagine this technology being useful at smaller, community hospitals where emergent laparotomy capabilities are not available,” Dr. King wrote. “A community surgeon could ‘foam’ a patient to provide hemostasis and transfer to a Level I center for definitive care…for a patient who would have otherwise bled to death on the way.”
Did you like this article? Our free monthly newsletter covers new technology and innovations in the world of trauma care. Click here to subscribe.